Talk:Membrane potential
| This It is of interest to the following WikiProjects: | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
Requested move[edit]
- I have merged the contents from the article "Transmembrane potential" into this article and transformed the former in a redirect. I suggest that this article - "transmembrane potential difference" - is moved to membrane potential, currently existing as a redirect, as "membrane potential" is the most often used term and as far as I know, it denotes the same. --Eleassar777 13:22, 14 May 2005 (UTC)
- Support Eleassar777 22:46, 14 May 2005 (UTC)
- Support. Eleassar777 is quite correct on all counts. --TenOfAllTrades (talk/contrib) 18:25, 15 May 2005 (UTC)
- Support ~~~~ ashleyisachild 19:18, 19 May 2005.
- Support—membrane potential is definitely the more commonly used of these terms. JeremyA 02:07, 20 May 2005 (UTC)
- Support absolutely correct Synaptidude 7 July 2005 00:38 (UTC)
This article has been renamed as the result of a move request. violet/riga (t) 21:28, 21 May 2005 (UTC)
dt=0[edit]
If you set dt=0 in the equation for capacitive current, then it doesn't eliminate the time dependance. Rather it gives an infinite capacitve current. You are dividing by 0. Maybe you mean to approximate the derivative by the differential dV/dt ~= deltaV=0.1V.
- Which still makes no sense. There should clearly be an estimate of the time involved.Peiter 13:59, 21 December 2006 (UTC)
Merging[edit]
If there is a merge, this article should redirect to bioelectromagnetism; not the other way around. - Omegatron 14:55, Jun 19, 2005 (UTC)
- I strongly object to this proposal: I don't care what happens to bioelectromagnetism (or bioelectricity) but membrane potential is a widely used term in the fields of biophysics and electrophysiology (see, for example, the 461,000 google hits). It is also used in many textbooks—for example Hille (ISBN 0-87893-321-2), which is considered by many to be the bible of biophysics. JeremyA 15:42, 19 Jun 2005 (UTC)
- I'm afraid I have to strongly disagree on that one. Membrane potential–as JeremyA notes–is a term used very heavily used in cell biology. Bioelectromagnetism is–I think–a related topic but I don't think there's enough overlap to warrant a merge. That article could use some cleanup, though. The scope of and definitions in that article aren't as clear as they could be. --TenOfAllTrades(talk) 16:20, 19 Jun 2005 (UTC)
- ITA with Jeremy . There is no way that this article should be merged with bioelectricity or biomagnetism. Membrane potential is a specific biophysical property of cells, while biomagnatism and bioelectricity, at least as discussed in the referenced wikipedia articles, are epiphenomona at best, and utter nonsense at worst. Those two articles are pretty much incomprehensible in any case. Furthermore, students, scientists of every ilk, including cell biologists, are going to look for the term "membrane potential" if they want to know about it. It is from membrane potential that all of these other phenomena (e.g. EEG) arise. I've deleted the merge request, because
- it's ridiculous on the face of it, and,
- I'm being bold
Synaptidude 7 July 2005 00:37 (UTC)
prior texts still to be edited and incorperated[edit]
I cut all of the following text from the original article. Basically there were several, well not factual errors exactly, but enough factual ambiguities, that I found it easier to re-write it from scratch. I kept the text below in case anyone objects and wants to talk about reinstating some of it.
Cells are surrounded by a plasma membrane, which defines their extent and acts as a barrier between the cells and their external environment, for example interstitial fluid or blood plasma. The membrane, as a result of its lipid bilayer structure and specific membrane proteins, is selectively permeable (the hydrophobic interior prevents the passage of both large polar molecules and ions) and therefore will only allow certain species through. This selective permeability allows asymmetric concentrations of ions to exist between the intra- and extracellular fluids. These differences can be chemical or electrical (i.e. the difference in charge between the inside and outside). Most cells maintain a “membrane potential” of around –80mV relative to the surrounding fluid. The membrane potential is negative because usually cells have a net negative charge due to leakiness of potassium channels and the large size of negatively charged macromolecules such as proteins and RNA.
In animal cells, passive ion movement accounts for the majority of the electrical potential across the plasma membrane. This passive ion movement mostly consists of K+ ions. A helps maintain an osmotic balance by keeping the concentration of intracellular Na+ low. Because the concentration of Na+ is so low inside the cell, other cations must be present to balance the negative charge carried by the cell's fixed protein anions. This balancing act is largely performed by K+ which is pumped in through the Na+/K+ pump and is also free to leave or enter the cell through the K+ leak channels. There is an electrostatic attraction for K+ due to the protein anions. This attraction balances against the tendency of K+ to diffuse out of the cell, down its concentration gradient, and it is these combined actions that create the membrane potential.
This can also be explained in the following way. Suppose that a cell initially has a membrane potential of zero – i.e. has no voltage gradient across the plasma membrane. However, the concentration of K+ inside the cell is higher than outside and so K+ will tend to leave the cell, driven by the concentration gradient. As it leaves the cell, the K+ leaves an unbalanced electrical charge. This creates a negative electrical charge, which is the membrane potential. The electrical field also opposes any further K+ leaving the cell. The membrane potential also tends to keep anions like Cl- out of the cell because their charge is also negative.
The cytosol, or interior, of a cell possesses a uniform electric potential or voltage compared to the extracellular solution. This voltage is the resting cell potential, also sometimes called the transmembrane potential of the resting cell. As an example, retinal ganglion cells have a resting cell potential of about -60 mV. Cells whose voltage is more negative than typical are said to be hyperpolarized, and those more positive are said to be depolarized. Healthy cells do not naturally hyperpolarize or depolarize except for brief intervals, for example during a nerve impulse or action potential. Among other roles, the cell potential acts as a reservoir for metabolic energy, which cells use to drive the transport of solute molecules across the membrane, to communicate with other cells and to trigger intracellular events.
Between the inside and outside of the cell (which is typically uniform electrically like the cytosol) the voltage rises very steeply just at the boundary created by the membrane. This create an electric field across the membrane, which exerts a force on ions and controls voltage-gated ion channels. Integral membrane proteins such as channels, pumps, and exchangers establish the membrane potential by transporting specific ions in or out. In essence, resting cells are negative because positively charged potassium ions, which are more concentrated inside than outside, are allowed to leak out. The resulting negative voltage difference between inside and out is therefore approximately equal to the reversal potential for potassium. Sodium-potassium exchangers maintain intracellular potassium at a high concentration while pumping sodium into the extracellular solution, where the concentration of sodium typically is high.
The Goldman equation can be used to calculate the membrane potential given the concentration of ions on either side of the membrane and their permeability.
Synaptidude 8 July 2005 22:17 (UTC)
I am a beginner student of membrane potential physiology and this removed text answered (mostly) the following questions I had more effectively then the article in its current form does:
1) What is the reason Na+ stays at its location right across the barrier on the extracellular side of the membrane? Diffusion? Electrochemical potential? Electric Charge repulsion?
2) If the answer is the electrochemical gradient/potential, because it is trying to move to a "less" positive potential.. if its located extracellularlly isn't that the "great unknown" where any molecules/cells/protein etc. are able to float by and possibly influence it? What happens if one of these has a "LESS" positive potential then the membrane that Na+ is currently attracted?
3) Can someone explain why can't Na+ be used for the entire process to establish the same voltage interactions leading from resting to action potential (as long as thier was less concentration intracellularly... if it is about LESS positivity ..Or vice versa for K+?)? — Preceding unsigned comment added by Spiked415 (talk • contribs) 15:39, 4 June 2012 (UTC)
Dissipation of ion gradiets by transmembrane ion flux[edit]
I removed the statement that action potentials run down the membrane ion concentration gradients. As I explained and calculated earlier in the article, the number of ions that cross the membrane during a large swing in membrane potential is infitessimally small compared to the ion concentrations supporting the gradients. Indeed it has been know since early days of studying action potentials, that upon disabling the sodium potassium pump, it takes many 10's of thousands of action potentials to run the gradient down appreciably. So while it is theoretically possible to run down gradients, it can really only happen under two conditions: 1) A highly unnatural experimental manipulation and 2) death.
voltage difference or potential difference?[edit]
I've struggled a little with this one myself. It has been changed to "voltage difference", but I'm not sure that is right. The voltage IS the difference between one side and the other of a resistor. One side of the resistor can't have a voltage, it is by definition a comparison between one side and the other. Still, I'm not sure "potential" is any better. In trying to think this through I'm considering the analogy to altitude. If you hold a ball 10 feet off the floor, the potential energy is the difference between the altitude of the ball and the altitude of the floor (just as voltage is the difference between one side and the other of a resistor). But the question is, what is the equivalent voltage term for the altitude of each thing (the floor and the ball)? I think "potential difference" comes closest, but what does anyone else think? Synaptidude 00:02, 25 August 2005 (UTC)
- See Electric potential. And for your analogy with gravity, see Gravitational potential. I think the technical term from physics is "potential difference" between the inside and the outside of a cell. This, from the fact that only differences in potential are important, not absolute values. Another point, maybe knit-picky, to keep in mind is that potential is NOT "potential energy", its potential energy per unit charge (for electrical potential) or per unit mass (for gravitation). I agree that we shouldn't use the term "voltage difference". One other point, people do sometimes talk about the voltage measured on one side of a resistor, but this is always in reference to an arbitrary 0 point (ground). In cell biology, often the fluid outside the cell used as the ground reference. I think this leads to somewhat sloppy terminology like "at rest, the cell is at -60mV".
- I'd agree, but add the nit-picky point that even in the case you mention (one side of a resistor), even if recording to an indifferent ground, you still won't get any voltage if there is no resistance between your measuring points. Good point on the /per unit comment. Thanks!Synaptidude 21:55, 25 August 2005 (UTC)
- Actually, this is kind of moot. I just went to the article to change it back to "electrical potential difference" from "voltage", but I realized that I'd misread it. It says "the electrical potential difference (voltage) across a cell's membrane...". This is a correct definition of voltage ("potential difference" = voltage). NEVERMIND! Synaptidude 21:59, 25 August 2005 (UTC)
- I'd agree, but add the nit-picky point that even in the case you mention (one side of a resistor), even if recording to an indifferent ground, you still won't get any voltage if there is no resistance between your measuring points. Good point on the /per unit comment. Thanks!Synaptidude 21:55, 25 August 2005 (UTC)
- This is an old discussion, but to clarify for anyone who stumbles across it: voltage is the difference in electrical potential between two points. Suppose we are given two unequal electrical potentials, A and B, and A != B. Since we said they're unequal, there is a potential difference between A and B that we might call A-B. Now let there be another potential, C. We can say there is a potential difference, A-C, between those two points. There is also a potential difference, B-C. And since A!=B, there is a potential difference between (A-C) and (B-C). This is potential difference is (A-C) - (B-C) which is A-B.
- Now you can substitute voltage for potential difference in the paragraph above. There exists a non-zero voltage at A relative to B. There exists a (possibly zero, since we never said A!=C) voltage at A relative to C. There exists a voltage at B relative to C. So you can say there is a voltage difference between A and B by comparing them both relative to C. In that statement, you are saying (A-C) != (B-C). Or you can say there is a potential difference between A and B, in which case you're saying A != B.
- Now let's say C is the reference ground potential, and further say that B is the outside of the cell, and that B=C, and that A is the inside of the cell. The voltage outside the cell (at B) is zero, since it is the reference ground (C). The voltage inside the cell (at A) is non-zero, since we said A!=B. There is a potential difference between the inside and outside of the cell. There is no potential difference between the outside of the cell and reference ground, because they are the same. Fhaigia (talk) 13:18, 9 November 2008 (UTC)
There are still basic errors in the definition of potential and the usage of electric potential vs. electric potential energy. The article states:
"Any voltage — membrane potentials included — is a separation of electric charges across a resistive barrier."
This is untrue - a voltage is a difference in electric potential (it is implied that this difference is between two points, or that it is with reference to some ground at which the voltage is defined to be zero). There is no need for a "resistive barrier" in order to have a voltage. Membrane potentials by definition DO require a resistive barrier - specifically, a membrane. I suggest removing/rewording the offending sentence. Later in this same section, it is parenthetically stated: "voltage is, after all, potential energy" This is untrue. Electric potential energy is a property of two (or more) charged particles. See http://hyperphysics.phy-astr.gsu.edu/Hbase/electric/elepe.html
The equation of electric potential energy is U = k*q*Q/r, where k is a constant, q is the charge of charged particle #1, Q is charged particle #2, and r is the distance between particles 1 and 2. The units of electric potential energy are commonly Joules or electron-volts - both , appropriately, units of energy. The equation for the electric potential due to a charged particle is V = kQ/r. Note the absence of a second charge, meaning that electric potential is not measured in the units of energy. Instead it is measured in units of energy per charge, or Joules/coulombs - also called volts. So it is not correct to say that the electric potential is potential energy. It would be appropriate to say that electric potential reports on the possibility of potential energy in the same way that height above ground reports on the possibility of gravitational potential energy. However, I would suggest doing away with the entire sentence and the ludicrously sketchy and inaccurate attempt at an energy pathway - I cannot fathom the utility of attempting to trace the path of energy from the sun to maintaining a cellular concentration gradient in one sentence.
Please let me know what you think, and if I am not dissuaded by feedback I will effect the changes I suggest above. Medphysicsguy (talk) 22:25, 20 November 2008 (UTC)
- I agree with this 100%. Well, 99% -- you can't really have a voltage difference for two points if the resistance between them is zero. But that's a quibble. Definitely I agree that it is a blunder to talk about energy here. looie496 (talk) 23:37, 20 November 2008 (UTC)
- Welcome to Wikipedia, by the way, since I see that this comment is your very first contrib. looie496 (talk) 23:40, 20 November 2008 (UTC)
- Thanks for the feedback. I'm making the changes. Just as a side note, it should be fine to have a potential difference between two points even if the resistance between them is zero. Consider a point charge, q, floating in a vacuum. Define two points at distances R1 and R2 from the charge. The potential difference between these two would be U2-U1 = k*q/R2 - k*q/R1. If R1 is not equal to R2, then the potential difference is non-zero, without any statement about a resistance between the two points. Probably not very useful a distinction to draw in real life, but in the realm of physics problems it's worth noting. Medphysicsguy (talk) 16:40, 25 November 2008 (UTC)
- I reworded "Any voltage — membrane potentials included — is a separation of electric charges across a resistive barrier" to the more accurate "Any voltage is a difference in electric potential between two points - for example, the separation of positive and negative electric charges on opposite sides of a resistive barrier."
- I also removed -
"In its most simple terms one can trace the origin of the energy of the membrane potential (voltage is, after all, potential energy) thus: Solar energy → converted to sugar by plants → converted by glycolysis to ATP → converted to concentration gradient by NaKATPase (sodium/potassium pump) → converted to voltage by passive K+ transport across a selectively permeable cell membrane."
- I did not replace it with anything, since tracing energy from the sun to the cell membrane is probably out of the scope of this article. Medphysicsguy (talk) 16:53, 25 November 2008 (UTC)
- I agree, and the edits look good. Concerning the other thing, the fact that you can't have a nonzero voltage difference with zero resistance follows from E=IR -- it would produce an infinite current. A vacuum actually has a very high resistance. looie496 (talk) 17:10, 25 November 2008 (UTC)
- Hmm... I definitely agree with you that in circuits, and in any real-world situation, there must be a resistance in order for a potential difference to be stably present. If there were a potential with no or very little resistance, then yes, a current would result until charges moved such that the electric field summed to zero (or such that any other forces were balanced by the electrostatic forces). However, the concept of resistance as I understand it is inherently materials science-based, and it was not invoked in any of my EM classes - we saved it for electronics. That is why I did not want to mention it in a basic definition of voltage (which is just a measure of potential). Also, Ohm's law applies to electrical circuits, and while there is a comparable equation for 3-d space and volume currents, it is not identical. And I don't remember what conditions must hold true in order to use the equation... Hmm. I think I need to refresh myself on this material. Thanks for your comments. Medphysicsguy (talk) 00:29, 30 November 2008 (UTC)
- So, hypothetically, what would the potential look like due to a point charge inside a superconducting material? I'm guessing you will say that the situation is an impossibility... but I am not familiar enough with this to know why. Medphysicsguy (talk) 00:29, 30 November 2008 (UTC)
- It's an impossibility on a macroscopic time/space scale. If you put a naked charge into a superconductor -- or the interior of a neuron for that matter -- it attracts charge from the surround, giving rise to a wave of charge movement that very rapidly ends with all the charge distributed evenly over the boundary (the membrane, for a neuron). Looie496 (talk) 01:22, 30 November 2008 (UTC)
- Right. But from your reasoning before that any potential difference must be across a non-zero resistance, this must mean that the resistance in the superconductor is nonzero, thus invalidating the premise of the hypothetical situation. It sounds like what you are really saying is that there is no non-zero resistance... even in a hypothetical? Medphysicsguy (talk) 01:47, 30 November 2008 (UTC)
- It's a question of time scale. On the finest time scale, ultimately you have to go back to Maxwell's equations, which don't directly involve a concept of resistance, as I recall (it's been quite a while since I've had to think on that level). But all this happens on a scale of nanoseconds or smaller. In neurons, where physiological processes happen on a time scale of milliseconds, all the basic electrical stuff can be treated as constantly in equilibrium. Looie496 (talk) 17:37, 30 November 2008 (UTC)
- Obviously, this discussion isn't relevant to physiological conditions or time scales. My questions were about the nature of an electric potential, since I had never heard the assertion that a varying potential field innately requires a resistance (outside of electronics, that is). You are correct that the basic equations of EM don't invoke resistance; hence, again, why I would never involve resistance in the definition of potential. In any case, thanks for engaging in this discussion. If I get a chance I'll try to look back at my EM books and see how they integrate the concepts of resistance and voltage. Medphysicsguy (talk) 23:47, 30 November 2008 (UTC)
- It's a question of time scale. On the finest time scale, ultimately you have to go back to Maxwell's equations, which don't directly involve a concept of resistance, as I recall (it's been quite a while since I've had to think on that level). But all this happens on a scale of nanoseconds or smaller. In neurons, where physiological processes happen on a time scale of milliseconds, all the basic electrical stuff can be treated as constantly in equilibrium. Looie496 (talk) 17:37, 30 November 2008 (UTC)
- Right. But from your reasoning before that any potential difference must be across a non-zero resistance, this must mean that the resistance in the superconductor is nonzero, thus invalidating the premise of the hypothetical situation. It sounds like what you are really saying is that there is no non-zero resistance... even in a hypothetical? Medphysicsguy (talk) 01:47, 30 November 2008 (UTC)
- It's an impossibility on a macroscopic time/space scale. If you put a naked charge into a superconductor -- or the interior of a neuron for that matter -- it attracts charge from the surround, giving rise to a wave of charge movement that very rapidly ends with all the charge distributed evenly over the boundary (the membrane, for a neuron). Looie496 (talk) 01:22, 30 November 2008 (UTC)
- I agree, and the edits look good. Concerning the other thing, the fact that you can't have a nonzero voltage difference with zero resistance follows from E=IR -- it would produce an infinite current. A vacuum actually has a very high resistance. looie496 (talk) 17:10, 25 November 2008 (UTC)
- I did not replace it with anything, since tracing energy from the sun to the cell membrane is probably out of the scope of this article. Medphysicsguy (talk) 16:53, 25 November 2008 (UTC)
Introduction[edit]
We need to tighten the introduction . It seems to be about three times longer than desirable. What do others think? David D. (Talk) 21:30, 25 January 2006 (UTC)
- Well David, this is about two years late, but you are correct, the intro definately needs to be tightened. Although I don't usually worry too much about the length, I have a serious problem with the first sentence.
- Membrane potential, is the electrical potential difference (voltage) across a cell's plasma membrane.
- I think when writing articles, it's always best to consider one's target audience to be high school students. I can understand using a complicated term like electrical potential because it's more accurate than voltage, but I never approve of using a term like "across". For someone who's not familiar with this field, "across a cell's plasma membrane" could mean a whole host of things. I think this should be reworded as:
- Membrane potential, is the electrical potential difference (voltage) of the extracellular solution from the electrical potential of the intracellular solution. Essentially it is the difference in voltage between the ions outside of the cell, and the ions inside of the cell.
- If I'm way off base as to what the membrane potential really is, then that supports my argument that this article is poorly introduced more so than anything. Paskari (talk) 20:21, 5 August 2008 (UTC)
- The (condensed) take-home message ("Essentially...cell.") reads "The membrane potential is the voltage difference between internal and external ions". This is not correct in physical terms. Correct is, that voltage is the difference between electrical potentials, not the other way around. Furthermore, "voltage between ions" is hard to percieve. Let me try this intro: "In biology, lipid membranes form fair insulators between electrically conducting compartments, e.g. inside and outside a cell. Correspondingly, the electrical potential difference (=voltage) between inside and outside is called membrane voltage, or more colloquial and somehow obsolete membrane potential." In case of no objections within the near future, I'll amend the text of the article correspondingly.Solfiz (talk) 13:40, 10 September 2008 (UTC) I
Electroneutrality!![edit]
I am no expert in biophysics at all, but during my chemistry degree "physical chemistry of biological processes" It was CLEARLY stated that both compartments (inside and outside) are electrically neutral, that means that there are many wrong sentences in the article, specially in the generation of the resting potential section. The electrical fields needed in order to generate a tiny deviation (10E-6 mol/liter from neutrality are millions of volts, clearly too much) The potential comes from the thermodynamic definition of electrochemical potential (chemical potential + electrical potential) and so and so.
I hope we can go through this but for the momento i'm putting on the expert tab, i think the article really requires rewriting by an expert
"Such a movement of one ion across the membrane would result in a net imbalance of charge across the membrane and a membrane potential"
"As potassium leaves the cell, it is leaving behind the anions" Those are clearly wrong, since cations must be accompanied by anions. An increase in permeability of a cation forces an increase of permeability in all anions, as the are forced to go with it through the membrane.
05:42, 5 November 2006 (UTC)Knights who say ni
I'll post my references in a couple of hours
(as a matter of fact there can be a slight local deviation of electroneutrality on the membranes due to their own electric load but that is not the cause of the potential ...)Knights who say ni 18:07, 17 September 2006 (UTC)
- What do you mean by "since cations must be accompanied by anions. An increase in permeability of a cation forces an increase of permeability in all anions" You don't seem to be considering that these ions have specific channels (more for some than others) and for some, whether they are open or not can be regulated. Or am i misunderstanding your point?
- Also this artical seem to be discussing the electrical potential ONLY. This is not synonymous with the electrochemical potential. What do you mean by "both compartments (inside and outside) are electrically neutral". If there is an electrical charge across the membrane how can both be electrically neutral? David D. (Talk) 04:46, 18 September 2006 (UTC)
- The opening of channels can be interpreted as an increase in average permeability of that ion. And that forces other ions with the opposite charge to circulate more, because there can't be a difference in charge across the membrane. The electrical charge across the membrane (which obviously exists) is very small compared to the causes of the membrane potential and is not taked into account when studying the system theoretically (for example in the Goldman equation Knights who say ni 07:30, 18 September 2006 (UTC)
I found this article confusing for this exact reason, and I have to say I support the expert tab for the moment. I'm worried that there is some confusion regarding terminology (which I can't clear up, because I'm not an expert), but here is what I do know: just two days ago I had a lecture (I'm doing a masters in neuroscience) wherein the teacher was careful to dispel the notion that the membrane potential is due to a difference in the charges inside and outside the cell. In fact he was very careful to point out that the charges are neutral. Like Knights who say ni says, the potential difference is due to the thermodynamic properties of any cell with ion concentration differences inside and outside the cell (see Goldman eqn which Knights linked, and also Nernst equation). Now, this may seem totally counterintuitive if we imagine that the membrane potential is an "electrical charge across the membrane" but it is not. Charge is measured in coulombs, and a difference in charges would also be measured in coulombs. This is distinct from voltage (though related). Voltage is a difference in electric potential, which indicates a non-zero electric field, i.e. there is a non-zero amount of force per coloumb in the area. I need to learn a lot more before I'm comfortable rewriting some of this article, but I think that it suffers from some very common misconceptions about what voltage is. (In my second year bio-psych course I was told the difference in charge was the cause, but I was told that by a psychologist who was no expert on cellular biophysics.) Tyrell turing 20:40, 2 November 2006 (UTC)
- I went and picked up a good text to figure out why this all seemed so weird to me (Principles of Neural Science, Kandel, Schwartz, and Jessell). I've discovered that I was confused in some ways, but right that the charges are neutral. I think the article does little to address my confusion though, or the confusion of people who think that the charges aren't neutral. Here's what I'd say about it: the article doesn't say anywhere that the charges aren't neutral, but it also doesn't spell out how the charge can be neutral when there is a seperation of charges across the membrane. From what I gather in the book, the article is right in that it is the movement of K+ ions across the membrane (as a result of the chemical gradient), that creates a separation of charge across the membrane, which creates a balancing voltage gradient (because voltage = charge/capacitance). This balancing voltage gradient eventually causes K+ ions to flow in, such that the overall net flow of K+ ions across the membrane is nil, thus reaching electrochemical equilibrium. Of course, although the numbers going in and out balance, there are still a few more positive ions outside, i.e. we still have a separation of charge across the membrane. However, what the article should spell out is that this small excess of positive and negative ions attract each other and line up along the membrane, such that the charges are 100% neutral everywhere except right across the membrane. I didn't find that clear reading it.Tyrell turing 20:48, 3 November 2006 (UTC)
- The key here is that the membrane is only permeable to some ions AND is a closed system. Clearly from above you do understand this. Try this reference. http://advan.physiology.org/cgi/content/full/28/4/139 it may help you more. If you can not access it, send me an e-mail and I'll respond with the pdf. David D. (Talk) 23:52, 3 November 2006 (UTC)
- It is vital to understand that all you say is obviously correct; BUT the thing is that whenever K+ ions are drawn in one direction, some Na+ or Cl- ions have to cross the membrane in order to balance the charge. So there can be an imbalane in the concentration of ONE ion because the other ones compensate for itKnights who say ni 05:14, 4 November 2006 (UTC)
- These ions (Cl- and Na+) do not necessarily move in response to movement of potassium since they cannot move if there are no open channels. Plus, it also depends on their concentration gradients across the membrane too. Are you really saying that for every potassium that leaves the cell a choride HAS to leave or a sodium HAS to enter the cell? Where have you read such a thing? David D. (Talk) 03:47, 5 November 2006 (UTC)
- It is vital to understand that all you say is obviously correct; BUT the thing is that whenever K+ ions are drawn in one direction, some Na+ or Cl- ions have to cross the membrane in order to balance the charge. So there can be an imbalane in the concentration of ONE ion because the other ones compensate for itKnights who say ni 05:14, 4 November 2006 (UTC)
- I'm clearly no expert, otherwise i would have just corrected everything i could prove wrong, The thing is that i don't understand all this well and the article really doesn't help.
There are statements about electroneutrality here [1] and here [2] and here [3] and here [4] Just type electroneutrality and membrane potential in google... The thing is the article needs an expert who can explain what goes on and cite a basic level source (a good old thick book), and hopefully have it sent to us in pdf... The article you cited before states that
These values are meant to represent only hypothetical values for a mammalian cell. Veq, calculated Nernstian equilibrium potentials for each ion gradient; Pi, “typical” permeability values for mammalian neurons. The listed concentrations of Cl� in the cytoplasm and extracellular fluid do not add up to the total concentration of K� and Na�. However, macroscopic electroneutrality is maintained in each compartment through the combined contribution of a diverse array of additional charged solutes (both anionic and cationic).
Knights who say ni 05:42, 5 November 2006 (UTC)
- Electroneutrality is not an axiom but a (very useful & successful) approximation, see e.g. https://doi.org/10.1007/s10008-011-1323-x
- In our cells, we have small flows of very specific ions passing through the cell membrane and directly changing the potential across it, which does violate electroneutrality.
- However, violations are limited to very small distances (see https://en.wikipedia.org/wiki/Debye_length) and very short timescales.
- It gets a bit paradoxical when you realise that the equations we use to describe such charge transfer (e.g. https://en.wikipedia.org/wiki/Goldman%E2%80%93Hodgkin%E2%80%93Katz_flux_equation) are usually derived under an assumption of electroneutrality, and so it's very easy to get confused when reading (or writing) about this.
- But the main thing to remember is that electroneutrality is an assumption we use to make analysis possible, not physically what's going on. Michael.Clerx (talk) 15:15, 13 September 2023 (UTC)
- Woops, bad example: The GHK equation does not assume local electroneutrality! Michael.Clerx (talk) 20:15, 13 September 2023 (UTC)
Merge?[edit]
There is a distinct article on resting potential. Maybe the bit about resting potential here can be moved to there? I'd do it, if I wasn't very busy with dutch wikipedia. Methoxyroxy 12:37, 2 November 2006 (UTC)
expert tag off[edit]
I'm finally more or less conviced and the article is mainly right (although the writing could be improved but that's not uncommon in wikipedian topics about chemistry). Maybe it should be noted that (i find that a bit esoteric) although there is an electric potential across the membrane there is no actual measurable difference in the global concentration of positive and negative ions across the membrane, that is, there is no actual charge excess in either size. That's because the effect of charge is hugely greater that the effect of concentration so an undetectable change in concentration creates a great change on electric potential. Knights who say ni 01:45, 24 November 2006 (UTC)
Big mistake?[edit]
It says "The typical membrane potential of a cell arises from the separation of potassium ions from intracellular immobile anions across the membrane of the cell." Isn't this a big mistake? The sodium-potassium pumps move sodium out of the cell and potassium in. True that the large anions (mainly proteins) are stuck inside the cell, but potassium ions are not separated from these anions--potassium is more concentrated on the inside of the cell than the outside. Should "sodium" simply be substituted for "potassium" in this sentence? I'm going to change it, and someone can "fix" it if they see this as a problem. Provophys 20:39, 26 July 2007 (UTC)
Thank you[edit]
This is a nice article, informative and well worded. Just wanted to say thanks to the peepz involved. Ft. Jack Hackett (talk) —Preceding comment was added at 21:41, 3 June 2008 (UTC)
I agree: this is a nice article. Thanks! Wtt (talk) 12:28, 23 January 2010 (UTC)
What is the membrane potential[edit]
No body really addresses what the membrane potential is, other times, like the first sentence in this article, it uses such a high level of language, no average person can understand it. Is the membrane potential the voltage of the cell's membrane, or is it a difference of the outside voltage-inside voltage? I don't like when people use phrases like "across membrane" or "against concentration gradient", if you're not familiar with this field, you won't understand what it means. Paskari (talk) 20:34, 5 August 2008 (UTC)
Reorganizing the field of MEMBRANE POTENTIALS[edit]
There are three articles now which discuss very much the same things with numerous repetitions and description of the same material in different terms.
As it was already suggested by Methoxyroxy 12:37, 2 November 2006 (UTC), it needs a really big clean-up and optimization. There is a lot of confusion there so I will do this albeit not at once. I will move different parts between these three articles, edit and unify their style etc. At later stage I will need someone who is native English speaker to do spellcheck.Rvfrolov (talk) 20:52, 2 January 2009 (UTC)
- I will be happy to do that when you are ready. I agree with you that these article need a lot of clean-up. Action potential was once a featured article, but it has become far too long and poorly organized. One thing to keep in mind is that, although the mathematics should certainly be treated, these articles should also give useful information to readers who cannot understand the math. Looie496 (talk) 21:59, 2 January 2009 (UTC)
Reorganizing the field of MEMBRANE POTENTIALS-2[edit]
The article will be messy for a while until i finish editing. It may take several daysRvfrolov (talk) 22:41, 2 January 2009 (UTC)
Sodium ion reverse potential[edit]
Sodium ion reverse potential should be around +60 mV rather than the 40 mV value previously reported in this article. I have also edited the intracellular sodium concentration. If it were 60-120 times more concentrated outside the cells compared to the inside the reverse potential would be more than +100 mV.
Prevention of leakage of the H+ and Na+ Transmembrane Potential by Sterols[edit]
this is an important and underappreciated aspect of the membrane potential and is becoming much studied by those concerned with long term effects of cholesterol reduction strategies and the effect on muscular organs like the heart. Glynwiki (talk) 08:06, 14 August 2009 (UTC)
ion number[edit]
i dont think that the page talks about the amount of ions that are being moved by the channels, it makes a slight hint that neutrality would take a while to re establish however. —Preceding unsigned comment added by Nibelhim (talk • contribs) 16:09, 16 February 2010 (UTC)
- Voltage gated channels24.203.202.14 (talk) 00:05, 17 February 2010 (UTC)
Moved from article[edit]
The part about voltage is not correctly written. Voltage is due to a barrier and a difference in charge.one side of the barrier might be high or positiv in charge, the other side must be the opposite in charge. If the barrier is zero in resistance no voltage will occur, because the current might flow freely. So if one wants voltage differece, one needs to create a barrier for currents. One might create a large barrier for current , voltage will then be maximal (open contacts) currents will be zero. But when a current runs due to a lower barrier voltage will drop.
Anthony (talk) 13:44, 12 September 2010 (UTC)
Notes from R.Bingelli[edit]
(The following material was inserted by R.Bingelli (talk · contribs) at the top of the article -- I am moving it here because it trashes the layout, and needs to be properly interwoven with the rest of the article. Looie496 (talk) 15:12, 18 August 2011 (UTC))
General Biological Properties of Membrane Potentials
I. Membrane Potential is a Basic Biological Property
Having a membrane potential (voltage) across the cell (plasma) membrane is a fundamental biological feature of all living true cells (eukaryotic cells). All vertebrate, and invertebrate animal cells as well as plant cells have a voltage difference between their exterior and interior with the interior usually negative with respect to the exterior. The voltages often range between 10 and 200 millivolts with plant cells usually having a somewhat larger voltage. 1,2,3
Because membrane potentials have primarily been extensively studied as a differentiated property in nerve cells of multicellullar animals, the general finding of a membrane potential in all other cell types tends to be neglected in most basic textbooks of biology.4,5 That this voltage is a fundamental property of all living cells, poses the urgent question of its more general or basic biological function in all of these other non-neuronal cell types.
1 Binggeli, R., & Weinstein, R. C. (1986). Membrane potentials and sodium channels: Hypotheses for growth regulation and cancer formation based on changes in sodium channels and gap junctions., Journal of Theoretical Biology, 123, 377–401
2 R. Chasan, (1992) The Plant Cell, MEETING REPORT, Excitation in Plant Membrane Biology
3 Wayne, Randy, (2009), Plant Cell Biology, From Astronomy to Zoology, Academic Press.
4 Sadava, D, Hillig, D.,.Heller, C., Bevenbaum, M. (2011) Life, The Science of Biology, 9th ed. Sinauer, W.H. Freeman & Co.
5 Wolfe, Steven (1992) Molecular and Cellular Biology, Wadsworth Publishing Co.
II. Within a Given Cell Type, Membrane Potential Size Differences are Correlated with the Cycle of Cell Division
In normal cells membrane potential values vary up and down. This not a random process but is usually correlated with the cell cycle. The voltage dips to very low values during cell division and rises again in the G1phase to stable values during the G0 phase. In in vitro tissue cultures when cells are newly seeded and dividing the average membrane potentials are very low. When cells come into contact their average potential rises and gradually stabilizes to its highest value as cell division ceases.6 Membrane potential is further correlated with terminal formation of normal tissue structures continuing to rise after cell to cell junctions are established.in proliferating cells (contact inhibition).7
6 Binggeli, R., & Weinstein, R. C. (1985) Deficits in elevating membrane potential of rat fibrosarcoma cells after cerll contact. Cancer Research 45, 235-241 7 Cone, C. D., Jr., & Tongier, M., Jr. (1973). Contact inhibition ofdivision: Involvement of the electrical transmembrane potential. Journal of Cellular Physiology, 82, 373–386.
III. Membrane Potential Varies with Mammalian Tissue Types
Membrane potential is correlated with stability or growth potential of various tissues. The voltages are higher in tissues that are mitotically stable, rarely divide, and are unable to repair by cell division after injury in higher mammals. e.g. muscle and nerve cells (- 70-90 millivolts). The membrane potential is lower (- 40-50 millivolts) in tissues that have a higher capacity for divisional repair after injury such as liver, pancreas, and thyroid. Other cell types have intermediate value (- 50-70 millivolts), kidney, glia, and smooth muscle.
1 Binggeli, R., & Weinstein, R. C. (1986). Membrane potentials and sodium channels: Hypotheses for growth regulation and cancer formation based on changes in sodium channels and gap junctions., Journal of Theoretical Biology, 123, 377–401
IV. Normal Resting Cells have Different Membrane Potentials than Proliferating Cells.
There is a boundary of membrane potential value which separates normal resting cells from normal proliferating cells and abnormal or neoplastic cancer calls and that value is approximately - 38 millivolts. Voltages larger than that tend to be in stable adult tissues. Voltages smaller than that tend to be in embryonic, infected, neoplastic, or proliferating normal calls in tissue culture.1,3 The notion that mitotic control and cancer formatrion were both related to membrane potential was first introduced by Clarence Cone in 1971.2 For confirmation it was further found that mitosis could be induced by depolarizing (making the voltage less) in normal calls.4
1 Binggeli, R., & Weinstein, R. C. (1986). Membrane potentials and sodium channels: Hypotheses for growth regulation and cancer formation based on changes in sodium channels and gap junctions., Journal of Theoretical Biology, 123, 377–401
2 Cone, C. D., Jr. (1971). Unified theory on the basic mechanism of normal mitotic control and oncogenesis. Journal of Theoretical Biology, 30, 151–181.
3 Binggeli R and Cameron IL.(1980) Cellular potentials of normal and cancerous fibroblasts and hepatocytes. Cancer Res 40: 1830–1835.
4 Cone, C. D., & Cone, C. M. (1976). Induction of mitosis in mature neurons in central nervous system by sustained depolarization. Science, 192, 155–158.
V. Natural Killer (NK) Cells Activity is Correlated with Membrane Potential.
Peripheral blood monocytes contain one cell type, Natural Killer (NK) cells which attack and kill cells which are infected with viruses, have neoplastic changes or cells that are still in a developmental or fetal stage, all without immunilogical priming. One study showed that the intensity of the attack on tissue culture cells was directly related to the membrane potential of the target cell regardless of its origin.1 Membrane potential in a cell may then be a primary determinant of vulnerability to natural killer cell surveillance and attack in mammalian systems.
1 Stevenson, D.,Binggeli, R.,Weinstein, R.C.,Keck, J.G.,Lai, M.C., Tong, M.J. (1989) Relationship between Cell Membrane Potential and Natural Killer Cell Cytolysis in Human Hepatocellular Carcinoma Cells. Cancer Research, 49: 4842-4845
VI. Recording Membrane Potentials.
Membrane potentials are a delicate property of living cells. Anything that affects the viability of those cells therefore affects the magnitude of the membrane potential since the latter is an excellent indicator of cell viability. Some cells are robust and tolerate rough handling while other cells are extremely dependent on such variables as temperature, pH, gas concentrations and detailed chemical environment. Given the proper environment, though, sure measurements are best made with direct electrical recordings through glass micropipettes coupled to a high impedance amplifier. Various other indirect methods using sensitive dyes for example cannot compare with the accuracy of electrical recordings.
Lack of explanation?[edit]
If far more potassium leaves the cell than the amount of sodium that enters, why doesnt't the cell get depleted of sodium? — Preceding unsigned comment added by Ingelix (talk • contribs) 13:33, 3 September 2011 (UTC)
- I don't understand the question. The cell is depleted of sodium, in the sense that the concentration of sodium ions inside a cell is always very low. Looie496 (talk) 16:27, 3 September 2011 (UTC)
- Although the intracellular concentration of sodium is low, it isn't zero, ever, and there is enough internal sodium to meet whatever needs the cell has. --Tryptofish (talk) 19:32, 3 September 2011 (UTC)
Cell Membrane Equivalent circuit.svg[edit]
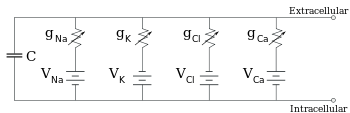
I'm not an expert on any of the areas that I'm discussing but something is not right with the schematic because it doesn't reflect the Goldman equation. First off, K^+, NA^+, and CA^+2 are all positively charged, the battery terminals should reflect that. Short bars are positive and long bars are negative. The circuit for CA^+2 is suspect since the Goldman equation is for monovalent ions. Maybe. — Preceding unsigned comment added by Pgrimes21 (talk • contribs) 11:15, 20 September 2011 (UTC)
- I believe the diagram is correct. K ions are indeed positive, but they have a higher concentration inside the membrane than outside, the reverse of the situation for Na and Ca ions. I don't think the fact that Ca ions are bivalent invalidates the diagram. Looie496 (talk) 14:52, 20 September 2011 (UTC)
- Speaking as an electrophysiologist but not an electrical engineer (so I'm pretty comfortable with the physiology, but less so with the conventions of drawing circuit diagrams), I also think the image is OK. Na+ is positively charged, and the direction that it moves across the membrane is from external to internal. K+ is also positively charged, but it moves in the opposite direction, and thus the opposite orientation of the symbol. Cl– moves in the same direction as Na+, but has a negative charge, so the symbol is oriented as for K+. Finally, Ca2+ moves in the same direction as does Na+, but as you say, it is divalent. If you take a look at the section of the page just following where the image appears, you will see that it says that the Goldman equation can also be expressed in a form that includes Ca2+, if desired. When this is done, the denominator becomes zF (as in the Nernst equation a bit higher up on the page), where z is the charge of the ion, in this case 2 for Ca2+ and 1 for everything else. (Actually, it's –1 for Cl–, but you'll notice that the ratio of logs is inverted there, which accomplishes the same thing.) --Tryptofish (talk) 19:33, 20 September 2011 (UTC)
Now I see, it's not about the charge, but about the flow from negative to positive. Thanks for clearing that up. — Preceding unsigned comment added by 69.76.20.175 (talk) 22:05, 20 September 2011 (UTC)
pumps and resting potential[edit]
A very nice article, thanks for the work. A small point, however. The 3rd para in the pumps section starts "A major contribution to establishing the membrane potential is ..." , then talks about the electrogenic nature of the pump, and then says "...thereby contributing to a positive voltage difference" This implies that the electrogenic aspect of the pump directly contributes significantly to the resting potential. My understanding is that the electrogenic bit only contributes a few millivolts If you poison the pump there is an immediate drop in resting potential of a few mV due to loss of the electrogenic thing, but then there is a gradual decline due to equilibration of the concentration gradient. (I think I read this in the Kandel and Schwarz big book some time ago - I don't know the original ref). But the important point is that the contribution of the pump to the resting potential is mainly indirect, working through its role in setting up the gradients. I'm new to Wikipedia, so I'm just posting this for discussion. Wjheitler (talk) 10:04, 23 October 2011 (UTC)
- The section you're referring to does not cite any source. As such, your making uncited changes isn't going to devalue the section in terms of trustworthiness. Please have your way with it if you're certain. If you think there's a good chance you're mistaken, best to leave it alone. If you're not certain but pretty sure the article is misleading, change it to retain the sound stuff but remove the stuff you're pretty sure is wrong. It is better to have no coverage of the point than potentially misleading assertions
- Ideal would be to do a little Google Books or Scholar search and find a source that settles the matter one way or the other. If you decide on this option, let me know the source and page number/s and I'll knock up the citation for you.
- If that doesn't interest you, you may be interested in the discussion just getting under way at Talk:Action potential about the merits of Action potential. --Anthonyhcole (talk) 10:39, 23 October 2011 (UTC)
- I think Wjheitler is probably right. Let me note, as the editor who wrote that section, that I've worked on this article because it is essential background for other articles and I thought it was important to have an explanation that a reasonably large group of readers could understand, but it isn't an area of expertise for me. I hope to have avoided huge blunders, but I'm sure that isn't the only error in the article. Looie496 (talk) 14:00, 23 October 2011 (UTC)
- That's correct. The immediate electrogenic effect of the pump is small and is due to the 3/2 ratio of sodium/potassium movement across the membrane. The major effect of the pump in establishing the membrane potential is in creating the concentration gradients. Synaptidude (talk) 15:08, 23 October 2011 (UTC)
- If I remember correctly, the Kandel and Schwartz book is a reference on this, but the membrane potential really results from separation of charge. The concentration gradient gets you part way there, but the differential permeability of the membrane means that, when those K+ ions go out, impermeant anions get left behind inside the cell. You don't generate voltage without separating charge. Resting (or "leak") K+ channels, combined with the impermeability of the membrane to large anions, are more important in this regard than pumps are. --Tryptofish (talk) 17:53, 25 October 2011 (UTC)
- correct. All voltages basically result from separation of charge. The concentration gradient is the second to last step in the process. Without it, you don't get net loss of K ions from inside the cell, and thus no voltage develops. But it's an essential part of the process. Without the pump, there's no concentration gradient. Without concentration gradient, there's no net movement of K. Without net movement of K, there's no membrane potential. I'm not telling you anything you don't know, except perhaps that I'd argue that pumps are just as important, just not as proximate. On a related note: this is what I'm thinking about doing: I kind of did what I wanted on the first paragraph - and of course you are welcome to edit. I'm thinking to leave the next couple paragraphs alone as they are general knowledge stuff. Then, for the benefit of non-experts, describe in a step-by-step (and illustrated) way the sequence pump ----> concentration gradient ---->selective movement of K down the concentration gradient = membrane potential. Then, I'd like to introduce the topic of why the membrane potential has the value it does. This would require the introduction of the concepts of driving force and permeability. This would require the introduction of the Nernst eq. and the concept of equilibrium potentials. Then move on to what happens when there is permiability to more than one ion. I realize this is sounding like it's getting pretty technical, but I think I can do it in an accessible way. Some of this involves moving stuff up that already exists further down in the article. Then finally, the details on ion channels and such comes at the end. Synaptidude (talk) 21:46, 25 October 2011 (UTC)
An Issue[edit]
Reading through the first part of this article, I'm having an issue with the definition of voltage. Voltage is a difference in energy between one place and another. It does not make sense to talk about the voltage at one location. In the first paragraph, the article talks about the membrane voltage being equal to (V interior - V exterior). But this doesn't make sense because there is no such thing as V interor and V exterior (it's also circular to define voltage in terms of voltage). You can't talk about a voltage at one place, because voltage is the *difference* between two places. The 'article' seems to understand this distinction because father down it says "but there is no instrument that can measure the voltage at a single point: the concept has no meaning". Yet throughout, the article talks about the different voltages at two points. The voltage *IS* the difference between the two points. There is no voltage at point A in a circuit and a different voltage at point B. There is a voltage between points A and B. In the case of membrane potential, there is no voltage on the outside and a different voltage on the inside, there is a voltage between the inside and the outside. Rather than define the membrane potential as Vm = (V interior - V exterior), why not instead use the equation Vm = (Zeuss - flying unicorns)? They are equally non-existent. (Sorry to be snide, I couldn't resist). Basically defining voltage this way would be like defining the distance that Bill is standing from Susan as 'the distance between Bill minus the distance between Susan'. It doesn't make sense.
There is also an attempt to define voltage in terms of it's ability to drive current. This is probably wise, but it ignores the role of resistance. It doesn't mention resistance. In fact, I can't find Ohm's law anywhere in the article which seems strange for an article on voltage, since Ohm's law IS basically the definition of voltage.
I think that the first part of the article should be re-written to give a more realistic definition of voltage, but it's hard to know what level to pitch it at. I understand that it is probably ill-advised to go back to the first-principles of thermodynamics and free energies to generate an accurate description, but we should be able to come up with something that is both accurate and understandable.
I would note that we had an extensive discussion on this topic several years ago in connection with this article, but it seems, since then, to have reverted to an incorrect definition of voltage.
-- On a smaller note, I deleted the line about the voltage-dependence of the NMDA channel that appeared under the 'voltage-dependent channels' heading. It was completely incorrect. The NMDA channel itself does not have appreciable voltage-dependence. Its apparent voltage-dependence derives from the voltage-dependence of magnesium blockage of the open channel, not from a voltage-dependence of channel gating, as had been stated. I thought about modifying the statement to reflect this, but then it became irrelevant to the topic of the heading, so I thought it best to just delete it. Synaptidude (talk) 15:22, 23 October 2011 (UTC)
This passage was also incorrect in calling the NMDA channel a 'calcium channel'. The NMDA channel is a mixed cation channel. It carries mostly sodium and potassium, and also some calcium. It is a calcium-permeable channel, but given that it is not selective for calcium and that a minority of the charge transferred through the channel is carried by calcium, it's not accurate to call it a calcium channel. If the group feels it is important to discuss ion channels that are gated by both ligands and voltage- perhaps the calcium-activated potassium channel would be a better example. But honestly, I'm not even sure why there are extensive descriptions of ion channels in this article. There are good wikipedia articles on ion channels and their function, and this 'membrane potential' article could be streamlined a lot by taking out the redundant description here and just referring to those articles where necessary. — Preceding unsigned comment added by Synaptidude (talk • contribs) 15:38, 23 October 2011 (UTC) Synaptidude (talk) 15:44, 23 October 2011 (UTC)
- Regarding Vinterior - Vexterior, you're correct of course, but every time I tried to remove that statement, somebody put it back, and eventually I just gave up and tried to edit around it rather than removing it. People have been taught that formula as the definition of membrane potential, and it's hard to unteach it. Regarding Ohm's law, the objective there was to say it in a way that would make sense to somebody who knows very little about electricity. If you see a better way to explain it, please take a shot. Regarding ion channels, I think this article needs to explain as clearly as possible what an ion channel is and the variety of things that can control them, but I agree that descriptions of specific types of channels are out of place here, except as examples. Looie496 (talk) 15:52, 23 October 2011 (UTC)
- Please dive in, Synaptitude. Most of the article is unsupported by sources, and if you're confident, just go ahead and make changes there. If you believe content that cites a reliable source needs changing, please cite a source that supports your change. The way to instill a degree of stability and permanence in your contributions is to cite (with page numbers) a reliable source (textbook, journal article, etc.) supporting them. Ask here for help constructing citations if you need it. In terms of accessibility, try to pitch it at a first year psychology student. Welcome back. --Anthonyhcole (talk) 16:21, 23 October 2011 (UTC)
I'm thinking that the section that deals with resting potential (the part containing the GHK equation) should move up to near the top of the article. It flows directly from the introductory paragraphs, especially as they are now written. The resting potential is not just potassium, but has smaller contributions from many ions, and I think it would be good to deal with this before one gets into the minutia about types of ion channels etc. I don't think that section needs much change, or expansion, as there is already a comprehensive article about resting potential, but more of a move. Synaptidude (talk) 05:00, 24 October 2011 (UTC)
- As elsewhere, I think the trick to aim for is to make it accurate while also keeping it brief and simple. I agree that voltage is a difference, and I think what confuses lay people is that the voltage across the membrane is indeed the difference in voltage between outside and inside, but that doesn't mean that there's one voltage inside and another voltage outside and you just have to subtract one from the other. Actually, the "exterior voltage" is just ground. I also agree that resistance and GHK are important. --Tryptofish (talk) 18:03, 25 October 2011 (UTC)
- I replied but somehow it ended up in the wrong part of the talk - I'm sure you'll find it. I'm also thinking that the first diagram needs to change. There are not enough negative charges in it, and I think that might be confusing to someone trying to suss it out. From the diagram, one might expect the inside of the membrane to be close to the outside in potential. Also, it's not clear what the triangles are. Ion channels? Gradients? Conceptually it's OK, but I think the 'math' needs to add up better. Also I think it could be better designed to show that the counter-ions are essentially across the membrane from each other (on the membrane capacitance). I know, I know, just dive in, and I will. Synaptidude (talk) 03:31, 26 October 2011 (UTC)
I made and put in a new figure 1. I tried to stay true to the style of the previous figure, but corrected incorrect 'ion math' and also embedded a more accurate visual image of the membrane potential (i.e. the relevant ions are right at the membrane). Also made the caption longer (maybe too long?) and more accurate. The previous caption said implied that K, Na and Cl all participate equally, when potassium is the dominant ion most of the time. I am planning on dealing with the issues of permeability and driving force, and what those mean to each ion's contribution to the membrane potential, later in the article. Synaptidude (talk) 21:18, 26 October 2011 (UTC)
Interactive tutorial proposed external link[edit]
I wrote an interactive tutorial "The origin of the resting membrane potential" which was intended as a teaching aid for my own students (UK university) but which is available for public view. I've had a number of nice e-mails from students (and some staff) in other universities who seemed to find it helpful, so I thought it might be appropriate as an external link on this page. In line with Wikipedia guidelines I'm putting this on the talk page, and inviting another editor to insert this link into the article if they feel it appropriate. I suggest the tag "Interactive Tutorial". http://www.st-andrews.ac.uk/~wjh/neurotut/mempot.html Wjheitler (talk) 11:19, 30 January 2014 (UTC)
Assessment comment[edit]
The comment(s) below were originally left at Talk:Membrane potential/Comments, and are posted here for posterity. Following several discussions in past years, these subpages are now deprecated. The comments may be irrelevant or outdated; if so, please feel free to remove this section.
| rated top as high school/SAT biology content - tameeria 14:50, 17 February 2007 (UTC) I'm not an expert on membrane potential, so please adjust the rating if B doesn't seem right. - tameeria 18:50, 18 February 2007 (UTC) |
Last edited at 18:50, 18 February 2007 (UTC). Substituted at 23:41, 29 April 2016 (UTC)
First paragraph[edit]
The introduction is a bit confusing. Why does it say "for the exterior of the cell"?
- C-Class vital articles
- Wikipedia level-5 vital articles
- Wikipedia vital articles in Biology and health sciences
- C-Class level-5 vital articles
- Wikipedia level-5 vital articles in Biology and health sciences
- C-Class vital articles in Biology and health sciences
- C-Class Molecular Biology articles
- Unknown-importance Molecular Biology articles
- C-Class MCB articles
- Top-importance MCB articles
- WikiProject Molecular and Cellular Biology articles
- All WikiProject Molecular Biology articles
- C-Class neuroscience articles
- High-importance neuroscience articles
- C-Class Chemistry articles
- Low-importance Chemistry articles
- WikiProject Chemistry articles
- C-Class Physiology articles
- High-importance Physiology articles
- Physiology articles about cellular physiology
- WikiProject Physiology articles



