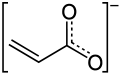
Acrylate
Acrylates (IUPAC: prop-2-enoates) are the salts, esters, and conjugate bases of acrylic acid. The acrylate ion is the anion CH2=CHCO−2. Often, acrylate refers to esters of acrylic acid, the most common member being methyl acrylate. These acrylates contain vinyl groups. These compounds are of interest because they are bifunctional: the vinyl group is susceptible to polymerization and the carboxylate group carries myriad functionalities.[1]
Monomers[edit]
Acrylates are defined by the formula CH2=CHCO2R, where R can be many groups:
- Acrylic acid
- Methyl acrylate
- Ethyl acrylate
- 2-Chloroethyl vinyl ether
- 2-Ethylhexyl acrylate
- Butyl acrylate
- Trimethylolpropane triacrylate (TMPTA)
The versatility of the resulting polymers is owed to the range of R groups.
- Structures of some acrylates
-
The acrylate anion
-
Trimethylolpropane triacrylate (TMPTA), a trifunctional acrylate ester
-
Methyl acrylate, an acrylic ester
-
Hexandiol diacrylate, a bifunctional acrylate
-
Pentaerythritol tetraacrylate (PETA), a tetrafunctional acrylate
-
A generic polyacrylate
Acrylate derivatives[edit]
Methacrylates ( CH2=C(CH3)CO2R) and cyanoacrylates ( CH2=C(CN)CO2R,) are closely related to acrylates. The feature a methyl and a nitrile in place of the H alpha to the carboxy functional group. They share several properties, being polymerized by radicals and being colorless.[2]
- Structures of some important modified acrylates
-
Methyl methacrylate, precursor to "perspex" (plexiglass)
-
Ethyl cyanoacrylate, precursor to "super glue"
Polymers[edit]

Some acrylate polymers (poly(methyl methacrylate) etc. not included):
Acrylate monomers are used to form acrylate polymers. Most commonly, these polymers are in fact copolymers, being derived from two monomers.[3][4]
Related polymers[edit]

In the same way that several variants of acrylic esters are known, so too are the corresponding polymers. Their properties strongly depends on the substituent.
A large family of acrylate-like polymers are derived from methyl methacrylate and many related esters, especially polymethyl methacrylate.
A second large family of acrylate-like polymers are derived from ethyl cyanoacrylate, which gives rise to cyanoacrylates.
Yet another family of acrylate-related polymers are the polyacrylamides, especially the parent derived from acrylamide.
Other uses[edit]
In addition to forming polymers, acrylate esters participate in other reactions relevant to organic chemistry. They are Michael acceptors and dienophiles. They undergo transesterification.
Production[edit]
Acrylates are industrially prepared by treating acrylic acid with the corresponding alcohol in presence of a catalyst. The reaction with lower alcohols (methanol, ethanol) takes place at 100–120 °C with acidic heterogeneous catalysts (cation exchanger). The reaction of higher alcohols (n-butanol, 2-ethylhexanol) is catalysed with sulfuric acid in homogeneous phase. Acrylates of even higher alcohols are obtainable by transesterification of lower esters catalysed by titanium alcoholates or organic tin compounds (e.g. dibutyltin dilaurate).[5]
See also[edit]
- Acrylate polymer
- Sodium polyacrylate thickeners
- Methacrylate
References[edit]
- ^ Takashi Ohara; Takahisa Sato; Noboru Shimizu; Günter Prescher; Helmut Schwind; Otto Weiberg; Klaus Marten; Helmut Greim (2003). "Acrylic Acid and Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_161.pub2. ISBN 3527306730.
- ^ Veerle Coessens; Tomislav Pintauer; Krzysztof Matyjaszewski (2001). Functional polymers by atom transfer radical polymerization. Vol. 26. pp. 337–377. doi:10.1016/S0079-6700(01)00003-X.
{{cite encyclopedia}}:|journal=ignored (help) - ^ Takashi Ohara; Takahisa Sato; Noboru Shimizu; et al. (2002). "Acrylic Acid and Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_161.pub2. ISBN 978-3-527-30673-2.(subscription required)
- ^ "Polyacrylates".
- ^ Arpe, Hans-Jürgen (2007). Industrielle organische Chemie: bedeutende Vor- und Zwischenprodukte (6 ed.). Weinheim: Wiley-VCH. ISBN 978-3-527-31540-6.